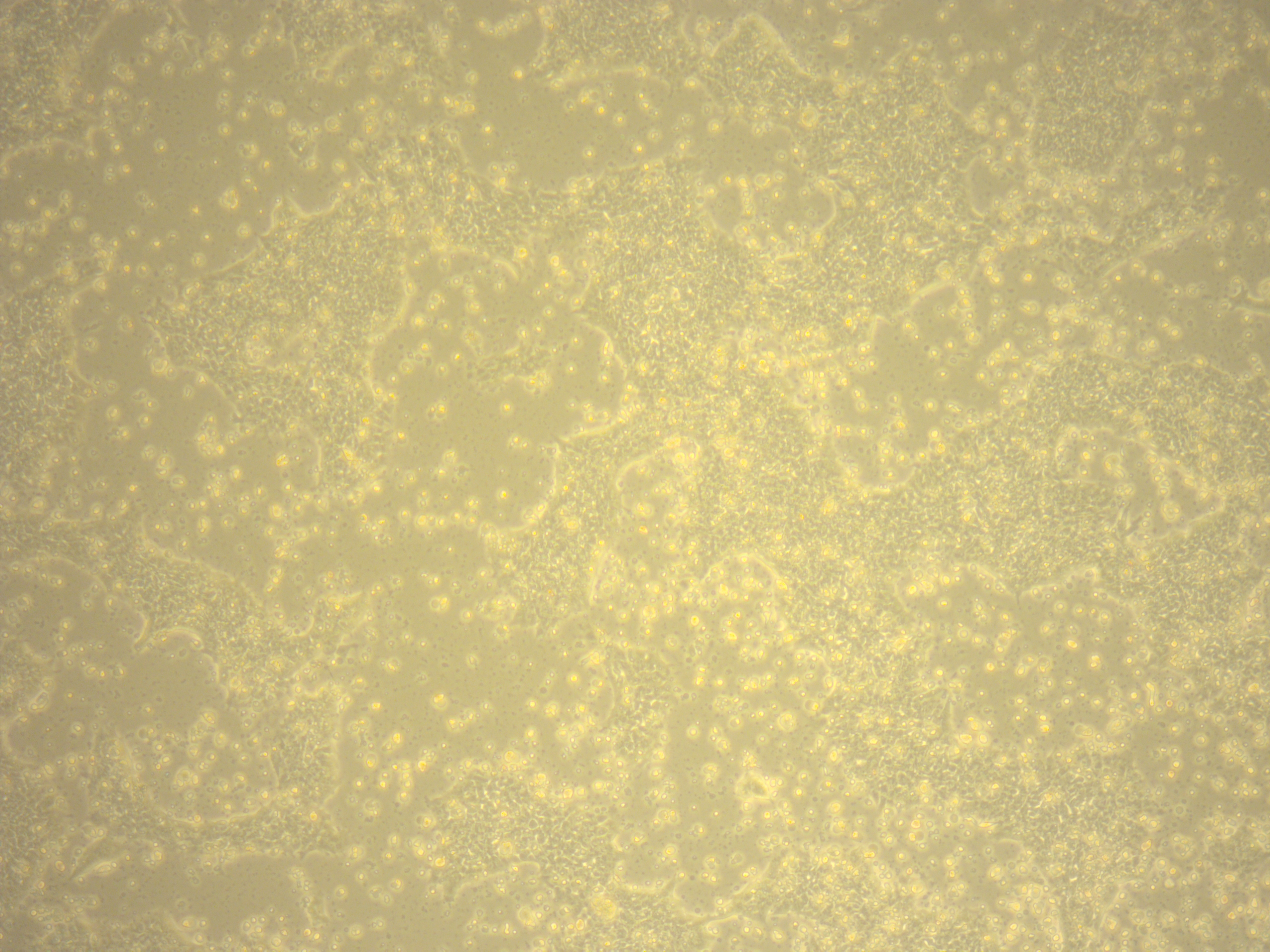
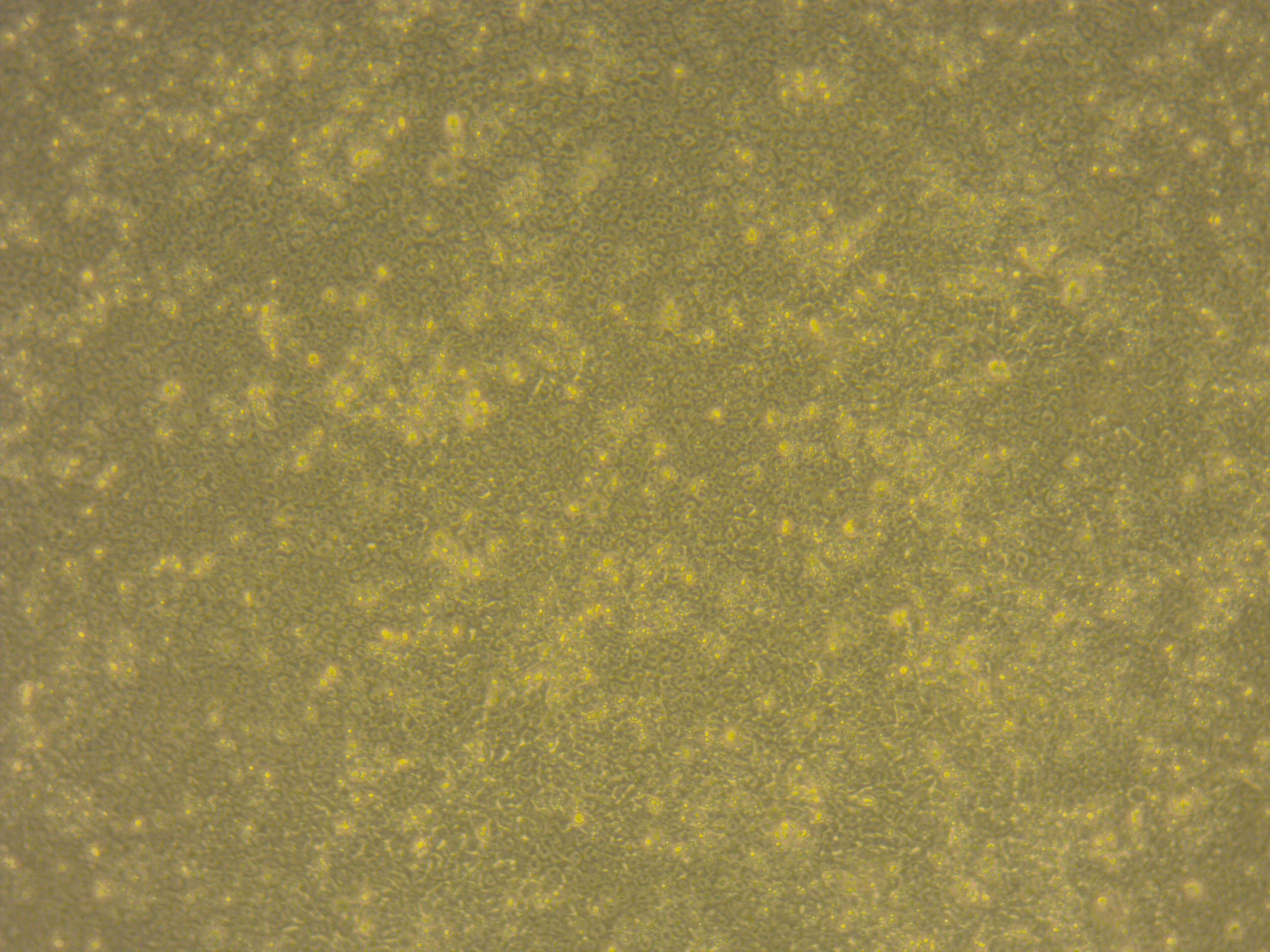
If the cells you would like to access are currently listed as unavailable
or you are ordering from outside of Europe please get in touch via
Contact@EBiSC.org.
SIGi001-A-11
iPSC0028 MAPT P301S+Ex10+16/Clone 7C6A4, SAMEA4451118
Gene-edited iPSC line
A CLIP contains information about a cell line including any
specific third party obligations relating to, for example,
licensing obligations or the donor consent which affect the
use of the cell line.
The EBiSC Access and Use Agreement must be completed along with an individual
Cell Line Information Pack for each line. Complete the EAUA and send to Contact@EBiSC.org
for countersignature. The EAUA must be fully signed before proceeding with your order.
A batch specific Certificate of Analysis will be available to
download once you receive your EBiSC iPSC line.
General#
Cell Line |
|
| hPSCreg name | SIGi001-A-11 |
| Alternative name(s) |
iPSC0028 MAPT P301S+Ex10+16/Clone 7C6A4, SAMEA4451118
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
SIGi001-A-8 (iPSC0028 MAPT P301S+Ex10+16/Clone 7D11A7) SIGi001-A-9 (iPSC0028 MAPT P301S+Ex10+16/Clone 7H6A1) SIGi001-A-10 (iPSC0028 MAPT P301S+Ex10+16/Clone 7G4A8) SIGi001-A-3 (iPSC0028 MAPT P301L C4) SIGi001-A-4 (iPSC0028 MAPT P301L D4) SIGi001-A-5 (iPSC0028 MAPT P301S 1C9C4) SIGi001-A-6 (iPSC0028 MAPT P301S 1B9C9) SIGi001-A-7 (iPSC0028 MAPT P301S 2G2B7) SIGi001-A-12 (iPSC0028 - BiAllelic MAPT_Ex10+16T/Clone 1F5-D12, SAMEA104237570) SIGi001-A-13 (iPSC0028 – MonoAllelic MAPT_Ex10+16T/Clone 1D01-11) SIGi001-A-16 (SIGi001-A-9 Bi-Allelic MAPT HA-tag) SIGi001-A-1 (iPSC0028 SLC17A7/GFP E3) SIGi001-A-2 (iPSC0028 SLC17A7/GFP C3) SIGi001-A-14 (SIGi001-A Bi-Allelic MAPT HA-tag) SIGi001-A-15 (SIGi001-A Dox inducible NGN2) SIGi001-A-17 (SIGi001-A-9 Dox inducible NGN2) SIGi001-A-18 (SIGi001-A-12 Bi-Allelic MAPT HA-tag #U28-12-P29-5) SIGi001-A-19 (SIGi001-A-12 Dox inducible NGN2) |
| Notes | The parental iPSC line SIGi001-A can be sourced via Sigma as 'iPSC0028'. |
Provider |
|
| Depositor | Sigma-Aldrich (SIG) |
| Distributors |
EBiSC
|
External Databases |
|
| hPSCreg | SIGi001-A-11 |
| BioSamples | SAMEA4451118 |
| Cellosaurus | CVCL_LE50 |
| Wikidata | Q54953432 |
General Information |
|
| This EBiSC line can be used for: |
Yes
Research use: allowed
Clinical use: no
Commercial use: no
|
| Subclone of | (not in EBiSC, see SIGi001-A in hPSCreg) |
Donor Information#
General Donor Information |
|
| Sex | female |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
| Family history | no |
| Is the medical history available upon request? | no |
| Is clinical information available? | no |
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
External Databases (Donor) |
|
| BioSamples | SAMEA4447434 |
hIPSC Derivation#
General |
|
|
More source cell information can be found in the parental cell line
SIGi001-A in hPSCreg.
|
|
Reprogramming method |
|
| Vector type | Integrating |
| Vector | Virus (Retrovirus) |
| Genes | |
| Is the used vector excisable? |
No |
| Absence of reprogramming vector(s)? |
Unknown |
| Reprogramming vectors silenced? |
Yes |
| Methods used |
RT-PCR
|
Vector free reprogramming |
|
| Type of used vector free reprogramming factor(s) |
None
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions#
Latest released batch |
|
| Culture medium | mTeSR |
| Passage method | EDTA |
| Surface coating | Matrigel / Geltrex |
| O2 concentration | 21 |
| CO2 concentration | 5 |
| Temperature | 37 |
The following are the depositor culture conditions, they do not refer to any specific batch.
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| O2 Concentration | 21 % |
| CO2 Concentration | 5 % |
| Medium |
mTeSR™ 1
|
Characterisation#
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|
||||
| SSEA-4 |
Yes |
|
||||
| TRA 1-60 |
Yes |
|
||||
| SSEA-1 |
No |
|
Differentiation Potency
In vitro spontaneous differentiation
In vitro spontaneous differentiation
In vitro spontaneous differentiation
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Sterility |
|
| Inoculation for microbiological growth | No Contaminants Detected |
| Mycoplasma | Not Detected |
| Viability | Viable post-cryopreservation |
Genotyping#
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
46,XX[17]/47,XX,+12[2]
Passage number: P37
Karyotyping method:
G-Banding
|
Other Genotyping (Cell Line) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Whole genome sequencing
https://ega-archive.org/studies/EGAS00001002755 This cell line has undergone WGS using the Illumina HiSeq X platform at 30x coverage. Fastq files are stored at the European Genome Archive, users can apply for access to this data by submitting an application form to the EBiSC Data Access Committee https://ega-archive.org/dacs/EGAC00001000768 |
Genetic Modification#
| Disease/phenotype related modifications |
There are three disease associations for the edited subclone: PSP (progressive supranuclear palsy) /CBD (Corticobasal degeneration) / FTDP-17 (Frontotemporal dementia and parkinsonism linked to chromosome 17) - like symptoms
Synonyms
Genetic modifications
MAPT (target)Isogenic modification
17q21.31
NM_016834.4 : c.[ c.727 >T; c.[741+16C>T]
Heterozygous
P301S+IVS10+16 C>T; both variants are heterozygous
Mutated
There are three disease associations for the edited subclone: PSP (progressive supranuclear palsy) /CBD (Corticobasal degeneration) / FTDP-17 (Frontotemporal dementia and parkinsonism linked to chromosome 17) - like symptoms
Synonyms
Genetic modifications
MAPT (target)Isogenic modification
17q21.31
NM_016834.4 : c.[ c.727 >T; c.[741+16C>T]
Heterozygous
P301S+IVS10+16 C>T; both variants are heterozygous
Mutated
Genetic modifications
MAPT (target)Isogenic modification
17q21.31
NM_016834.4 : c.[ c.727 >T; c.[741+16C>T]
Heterozygous
P301S+IVS10+16 C>T; both variants are heterozygous
Mutated
|